Disease/Disorder
Definition
Persistent tendon pain and dysfunction is related to mechanical loading.1 Encompasses tendinitis, tendinosis, paratenonitis, and tendon ruptures.2 Tendinitis typically refers to acute tendon injuries. Tendinosis refers to chronic, degenerative tendinopathy. Paratenonitis is inflammation of the areolar tissue surrounding the tendon.2
Etiology
Overuse with poor or altered mechanics. Contributing factors include an altered healing response, relative ischemia, apoptosis of tenocytes and changes in neuronal homeostasis leading to stimulation of nerve endings and mast cells which alters the tendon matrix.2 Repeated mechanical stress and recurrent injuries with an inappropriate inflammatory process may lead to the development of angiofibroblastic hyperplasia.3
Epidemiology including risk factors and primary prevention
- Most often occurring in 30- to 60-year-olds.
- Prevalence increases with age and is higher in women compared to men.4
- The most common upper extremity tendinopathies occur at the shoulder (i.e., supraspinatus) and elbow (i.e., common flexor and extensor tendons).
- The most common lower extremity tendinopathies occur at the heel (i.e., plantar fascia and Achilles tendon), greater trochanter (i.e., gluteus medius and minimus), knee (i.e., patellar tendon) and ankle (i.e., tibialis posterior tendon).
- Risk factors may be divided into intrinsic and extrinsic factors.4
- Intrinsic risk factors:
- Medical and metabolic disorders (diabetes mellitus, obesity, hyperlipidemia, seronegative spondyloarthropathies)
- Inflammatory conditions
- Family history
- Age
- Limited or excessive joint mobility
- Muscle weakness or deficits in neuromuscular control
- Altered tendon structure
- Polymorphisms in genes: collagen, type V, alpha 1 (COL5A1), tenascin C (TNC), matrix metalloproteinase-3 (MMP3), and estrogen-related receptor alpha (ESRRA) show highest association with tendon injury (i.e., tendinopathy or rupture)
- Extrinsic risk factors:
- Overuse
- Sudden increase in activity frequency and/or intensity
- New physical activities
- Lack of adequate recovery
- Repetitive movements
- Poor ergonomics
- Treatment plans using fluoroquinolone, excess corticosteroid use, and statins
- Intrinsic risk factors:
Patho-anatomy/physiology
- Normally, tendon fiber bundles are composed of fascicles consisting of tropocollagen (triple helix polypeptide chain) and blood vessels arranged parallel to the fascicles. The portion of the tendon that bears stress during mechanical loading is composed of three main components: Type 1 collagen, cells (mainly fibroblasts), and a non-collagenous matrix.3
- Pathologic changes include macrostructural thickening and increased vascularity. Microstructure changes include degeneration and disorganization of collagen fibers, increased cellularity, minimal inflammation, lengthening and decreasing volume of tenocytes, and increased Type III collagen density.4 The build-up of mucopolysaccharide in fibrous tendon sheath leads to mucoid (i.e., myxoid) degeneration. This cycle repeats, leading to globular degeneration and the production of matrix metalloproteinases (MMPs)5, tenocyte apoptosis, chondroid metaplasia of the tendon, and expression of protective factors such as insulin-like growth factor 1 (IGF-1) and nitric oxide synthetase (NOS), causing recurrent and chronic pain.6 Additionally, there is increased COX-2 and IL-6 expression indicating a low-grade inflammatory response.
- Upregulation of proteins, such as B-cell lymphoma interacting protein 3(BNIP3), implicated in pro-apoptotic pathways that promote oxidative injury, may play a role in promoting tendinopathy.3
Disease progression including natural history, disease phases or stages, disease trajectory (clinical features and presentation over time)
- An acute traumatic or insidious event may present as a tendinitis with inflammation of the tendon or paratenon, followed by a blunted inflammatory process or as an acute tear, often related to unaccustomed activity or a single instance of exertion. The healing process may be altered due to relative ischemia or other factors.
- The subacute or chronic nature of tendinopathy repeats the blunted inflammatory cycle resulting in a thickened and degenerated tendon susceptible to pain or an acute tear.4
- Recovery from tendinopathy can take up to 6-12 months or longer; however, those with less severe symptoms and tendon structural changes may have shorter recovery.4
Specific secondary or associated conditions and complications
- A variant of tendinosis (i.e., calcific tendinitis) is hydroxyapatite crystalline deposition into the tendon, often affects the supraspinatus (Figures 1 and 2) and progresses through stages including fibrocartilage ingrowth, calcium formation, resorption, and healing. Calcific tendinopathy is most painful during the resorptive phase and improves during tendon healing.
- Fatty infiltration can occur as the degenerated tendon vacuolizes, and space is filled presumably with proliferation of areolar tissue in the adjacent paratenon.
- Tendon rupture can be a complication of rapid and/or forceful eccentric strain in the setting of advanced tendinopathy. Rupture can sometimes be the initial event signaling the presence of tendinopathy (e.g., Achilles tendon).
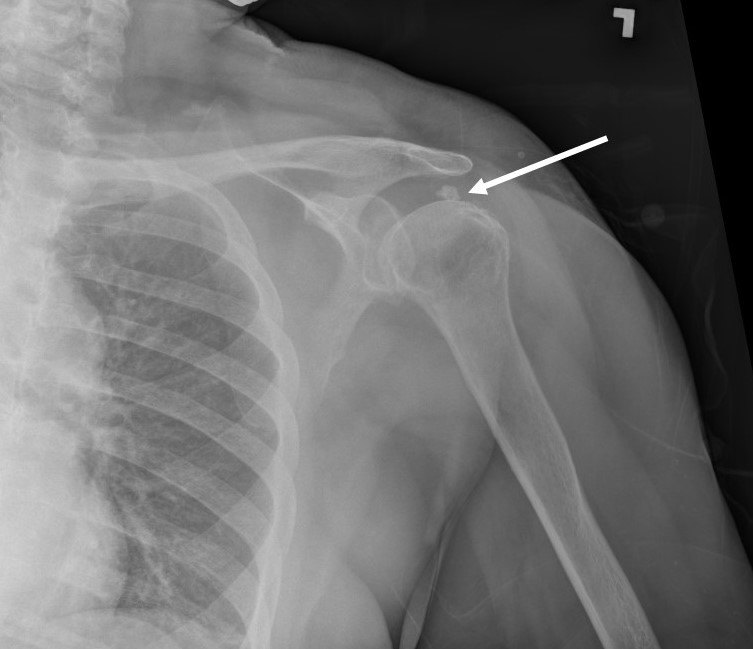
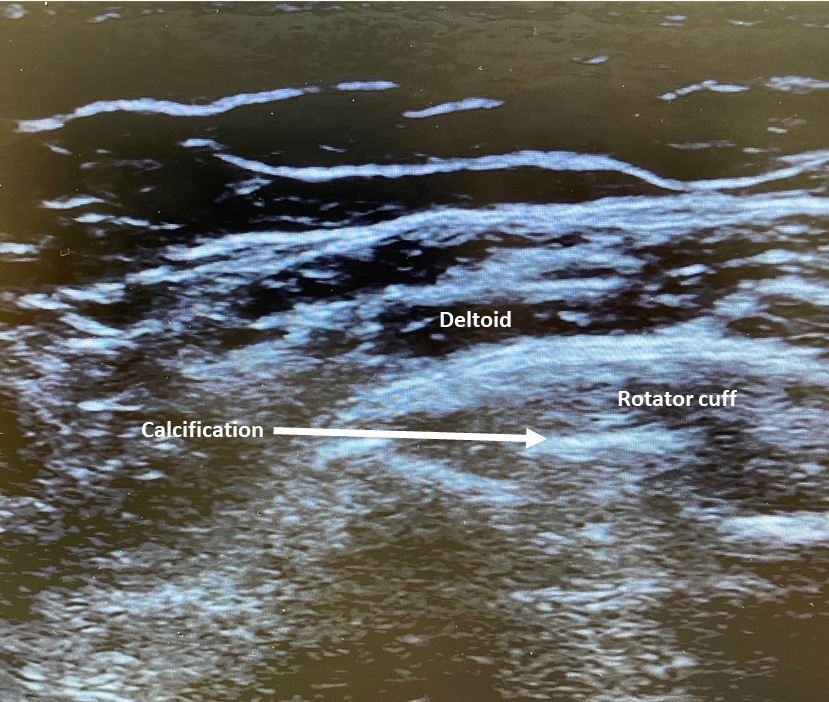
Essentials of Assessment
History
- Soreness and stiffness are often reported in the morning or after being still for long periods.4 Pain is often described as dull, aching, and sharp at times. It is usually focal and improves with rest, ice, and nonsteroidal anti-inflammatory drugs (NSAIDS), but may be exacerbated with use of the affected area.
- Initially, patients may note temporary resolution of symptoms after warming-up the affected joint.
- Important history includes prior injury of the affected limb, repetitive use, systemic illnesses, and recent medications, such as fluoroquinolones.
- Others present with history of a traumatic or non-traumatic acute rupture, with sudden inability to lift the affected limb overhead.
- In patients with progressive tendinopathy, pain may be debilitating during activities.
Physical examination
- Inspection is often normal but can reveal fullness or focal thickening over superficial tendons. Observing postural alignment during activities and biomechanics are important to identify predisposing factors.
- Palpation reveals tenderness anywhere from the enthesis to the myotendinous junction. Adjacent soft tissues or bursae may also be tender.
- Range of motion (ROM), active and passive, may be decreased or elicit end-range pain (e.g., elbow extension lag in severe lateral epicondylitis).
- Neurologic exam should be normal. Strength deficits, if present, should be pain-limited only.
- Special tests that can reproduce pain include passive stretching of affected tendon or resisted active motion of the tendon.
Functional assessment
- Evaluate for functional activities and ROM of adjacent joints (at least one joint above and below the lesion).
- Evaluate the posture, form, biomechanics, repetitive motions, or other issues that may promote a recurrence in athletes or workers. Workplace or ergonomic evaluation may also be helpful.
- Fully evaluate the kinetic chain for ROM deficits, strength, alignment, mechanical, and functional issues.
- Evaluate for pain during certain movements through provocative maneuvers.
- Single leg calf-raises or hopping for Achilles tendinopathy
- Single leg squats for patellar tendinopathy
- Resisted extension of the wrist or gripping for elbow tendinopathy
Laboratory studies
- May be necessary when multiple sites of tendon pain are identified to look for rheumatologic conditions.
- Association of positive Human Leucocyte Antigen (HLA)-B27 with bilateral Achilles tendinopathy.
Imaging
- Imaging is not necessary for clinical diagnosis of tendinopathy.1
- Radiographs may help determine the presence of intra-tendinous calcifications, calcified tendon insertions (enthesophytes), or concomitant articular or bone pathology, such as fracture or apophysitis.
- Magnetic resonance imaging (MRI) is generally not indicated unless grading a tear is necessary for advanced treatment or evaluation of adjacent structures.
- Musculoskeletal ultrasound is an efficient, noninvasive, and relatively low-cost dynamic imaging modality for tendinopathy.7 There are a variety of findings on ultrasound for identifying degenerative tendon changes which includes bone irregularities, calcific deposits, thickening, swelling, tendon tears, and neovascularization.
Supplemental assessment tools
Differential diagnosis
- Local inflammatory causes such as bursitis, synovitis, or apophysitis. The incidence of primary bursitis is disputed, but studies of greater trochanteric pain suggest true bursal distension is uncommon and unlikely to occur in the absence of gluteus medius tendon pathology.8
- Local degenerative causes such as arthropathy, cartilage injury, muscle/tendon tears, or other intra-articular pathology.
- Tumors, infection, or vascular causes.
- Referred or radicular pain.
Early predictions of outcomes
Functional outcome measures, some joint specific, can be utilized to chart the progress, or lack thereof, of treatment.9 Instruments to measure strength distal to the affected tendon over time can demonstrate treatment effects.
Environmental
Occupational settings that include forceful activities with high force and/or repetition, such as food industry workers, construction workers, and assembly line packers, increase the risk for developing tendinopathy.4
Social role and social support system
- Persons with tendinopathy may suffer depression or other mood disorders if they cannot participate in vocational or avocational activities. In such cases, activity adaptations or appropriate multidisciplinary treatment is required.
- Addressing psychosocial conditions that may precipitate depression and anxiety may improve outcomes in patients with tendinopathy since these psychological conditions correlate with pain.4
Professional issues
Patients with chronic recalcitrant tendinopathy may seek out unproven treatments, such as acupuncture, magnets, and other alternative or “naturopathic” treatments, and should be counseled appropriately on treatments that have scientific validity.
Rehabilitation Management and Treatments
Available or current treatment guidelines
Conservative management focuses on decreasing tendon load, followed by progressive loading at different disease stages.
New onset/acute tendinopathy
- Initial treatment should be tailored to the patient’s needs or desire to return to activity, symptoms, and the severity of tendinopathy.
- Individualized rehabilitation that incorporates strengthening progression for longer than 12 weeks should be prescribed as first-line treatment for tendinopathy4. Acute exacerbations may be managed with relative rest, NSAIDs, and physical modalities, including ice.
- Physical therapy may include friction massage, biomechanical corrections, and ergonomic adjustments.
- Isometric & eccentric exercise, patellar strapping and taping, dry needling (DN), and injections have a short-term pain reducing effect in addition to functional improvements.10 Topical aspirin or diclofenac may be helpful and have a small amount of systemic absorption. Glyceryl trinitrate patch may decrease pain and enhance healing.11
- Tendon sheath corticosteroid injections could give temporary partial pain relief, though use is controversial. Intra-tendinous injections are not recommended, as they may result in tendon tears.
- Corticosteroid injection of the rotator cuff decreases cell proliferation, alters collagen and extracellular matrix composition, impedes inflammatory pathways, decreases cell viability, and increases apoptosis. The evidence supports limiting a body part to 3 corticosteroid injections per year and waiting at least 1 month after injection before arthroscopic rotator cuff repair. Tendons exposed to steroids have a decreased load to failure and decreased strength in the early post-injection period.12
Subacute/chronic
- Tendinopathy is commonly recognized in the subacute or chronic stage.
- Other rehabilitation strategies include preserving adjacent joint ROM, improving flexibility or kinetic chain deficits, and optimizing function to return to activity.
- An eccentric strengthening program has been demonstrated as potentially curative for Achilles tendinopathy and patellar tendinitis.10,13
- Patient education, activity modification, and regaining tendon length to enable normal wrist extensor dynamics promote long-term function and pain improvements for lateral epicondylitis.14
Prevention
Primary prevention for tendinopathy includes adequate ergonomics and introducing exercise regimens that improve strength and coordination of muscle tendon units that may be predisposed to overuse-related tendinopathy.
Coordination of care
- Physical and occupational therapy, vocational counseling, and rehabilitation psychologists can all play a role in treatment.
- In cases of tendon tear, rupture, or calcific tendonitis, a surgical consultation or debridement/repair of severe tendinopathy may be necessary.15
Patient & family education
- Family and societal roles may change due to disabling conditions.
- Patient should be reassured that pain is allowed during and after performing exercises since sufficient tendon loading is necessary to cause meaningful clinical change.4
Emerging/unique interventions
Impairment-based measurement
Patients with work limitations may require functional capacity evaluations to quantify their work tolerance and provide objective work modification recommendations.
Measurement of patient outcomes
Joint specific functional outcome measures, when needed.9
Translation into practice: practice “pearls”/performance improvement in practice (PIPs)/changes in clinical practice behaviors and skills
Physicians may learn and integrate into their practice newer techniques described to treat tendinopathy, including biologics (platelet-rich plasma (PRP), autologous blood, stem cells, and other products).16
Cutting Edge/Emerging and Unique Concepts and Practice
- Injections under ultrasound and fluoroscopic guidance (Figure 3) increase the accuracy and efficacy of injections.17 Ultrasound provides superior visualization of soft tissue structures (i.e., tendons) without ionizing radiation.
- Injection of biologics (PRP, autologous blood, and stem cells) offers an alternative treatment for those who have failed conservative management.11, 16
- Extracorporeal shockwave therapy (ESWT) has demonstrated marginal or no significant symptom or functional improvements in patellar tendinopathy. ESWT has demonstrated favorable calcium resorption rates in rotator cuff calcific tendinitis but inferior to ultrasound-guided needling.13,18

Emerging/unique interventions
- Interventions directed at restoring normal tendon anatomy such as PRP, amniotic growth factor injections, and stem cell therapy are emerging treatments.
- In addition to promoting type I collagen gene expression, in vivo and in vitro studies of PRP demonstrate proliferation and organization of fibroblasts and tenocytes, confirming the positive effects of PRP on tendon pathology at a cellular level.19
- PRP therapy alone has been shown to be effective for lateral epicondylitis and has superior long-term benefits on patient outcomes in patellar tendinopathy.16
Gaps in the Evidence-Based Knowledge
- There is preliminary evidence to support the beneficial effects of blood flow restriction training (BFRT) on pathologic tendons, nevertheless more research is needed.20
- The most appropriate protocol for deploying PRP is not well defined. Many studies used different protocols with various amounts of biologic products and growth factors.16
References
- Scott, A. et al. ICON 2019: International Scientific Tendinopathy Symposium consensus: clinical terminology. Br. J. Sports Med 2020; 54: 260–262
- Raney EB, Thankam FG, Dilisio MF, Agrawal DK. Pain and the pathogenesis of biceps tendinopathy. Am J Transl Res. 2017;9(6):2668-2683.
- Scott, A, Backman LJ, Speed C. Tendinopathy: update on pathophysiology. J Ortho Sports Phys Ther 2015: 45(11): 833-841.
- Millar, N.L., Silbernagel, K.G., Thorborg, K. et al. Tendinopathy. Nat Rev Dis Primers 2021: 7: 10.
- Jones GC, Corps AN, Pennington CJ, Clark IM, et al. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human Achilles tendon. Arthritis Rheum. 2006; 54:832-842.
- Szomor ZL, Appleyard RC, Murrell GA. Overexpression of nitric oxide synthases in tendon overuse. J Orthop Res. 2006; 24:80-86.
- Vincenzo Ricci V, MD, Allison Schroeder A, MD, and Levent Özçakar L. Ultrasound Imaging for Lateral Elbow Pain: Pinpointing the Epicondylosis. Am J Phys Med Rehabi. 2020; 99(6): 560-561.
- Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. British Journal of Sports Medicine. 2017; 51(2): 97–104.
- Newcomer K. Martínez-Silvestrini JA. Gay R. White K. A Comparison of the Patient-Rated Forearm Questionnaire with Other Outcome Measurement Tools for Lateral Epicondylitis. Journal of Hand Therapy 2005; 18(4):400-405.
- Vander Doelen, T, Jelley,. Non-surgical treatment of patellar tendinopathy: A systematic review of randomized controlled trials. J Sci Med Sports 2020; 23(2): 118–124.
- de Vos RJ, Weir A, Cobben LP, Tol JL. Tol The value of power Doppler ultrasonography in Achilles tendinopathy: a prospective study. Am J Sports Med. 2007; 35(10): 1696–1701.
- Puzzitiello RN, Patel BH, Forlenza EM, et al. Adverse Impact of Corticosteroids on Rotator Cuff Tendon Health and Repair: A Systematic Review of Basic Science Studies. Arthroscopy, Sports Medicine, and Rehabilitation 2020; 2(2): e161–e169.
- van der Vlist AC, Winters M, Weir A, et al. Which treatment is most effective for patients with Achilles tendinopathy? A living systematic review with network meta-analysis of 29 randomised controlled trials. Br J Sports Med. 2021;55(5):249-256.
- Martínez-Silvestrini JA. Newcomer K. Gay R. White K. A clinical trial of concentric and eccentric strengthening for chronic lateral epicondylitis. Journal of Hand Therapy. 2005; 18(4):411-419.
- Drummond Junior M, Ayinon C, Rodosky M, Vyas D, Lesniak B, Lin A. Predictive factors for failure of conservative management in the treatment of calcific tendinitis of the shoulder. JSES Int. 2021; 5(3):469-473.
- Andriolo L, Altamura SA, Reale D, et al.,. Nonsurgical Treatments of Patellar Tendinopathy: Multiple Injections of Platelet-Rich Plasma Are a Suitable Option: A Systematic Review and Meta-analysis. Am J Sports Med 2019; 47(4): 1001–1018.
- ElMeligie MM, Allam NM, Yehia RM, Ashour AA. Systematic review and meta-analysis on the effectiveness of ultrasound-guided versus landmark corticosteroid injection in the treatment of shoulder pain: an update. J Ultrasound. 2023; 26(3): 593-604.
- Al-Abbad H, Allen S, Morris S, et al. The effects of shockwave therapy on musculoskeletal conditions based on changes in imaging: a systematic review and meta-analysis with meta-regression. BMC Musculoskelet Disord. 2020; 21(1): 275.
- Chalidis B, Givissis P, Papadopoulos P, Pitsilos C. Molecular and Biologic Effects of Platelet-Rich Plasma (PRP) in Ligament and Tendon Healing and Regeneration: A Systematic Review. Int J Mol Sci. 2023; 24(3): 2744.
- Burton I, McCormack A. Blood Flow Restriction Resistance Training in Tendon Rehabilitation: A Scoping Review on Intervention Parameters, Physiological Effects, and Outcomes. Front Sports Act Living. 2022; 4:879860.
Original Version of the Topic
Chris Visco, MD. Tendinopathy. 4/5/2013
Previous Revision(s) of the Topic
Julio Martinez-Silvestrini, MD, Hans Knopp, MD. Tendinopathy. 4/4/2017
Julio Martinez-Silvestrini, MD, Alvaro Nava, BSPH, MS, Christopher Elmore, MD. Tendinopathy. 4/29/2021
Author Disclosures
Julio Martinez-Silvestrini, MD
Nothing to Disclose
Samantha Sabban, DO
Nothing to Disclose
Elijah How, DO
Nothing to Disclose
